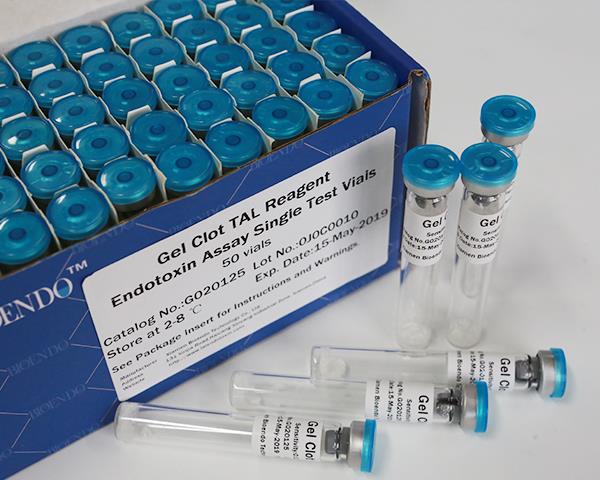
Limulus Amebocyte Lysate Assay LAL Reagent Single Test Vial Gel Clot Method
-
Min Order
1
-
Product Unit
Pieces
-
Origin
China Mainland
-
Payment


- Contact Now Start Order
- Favorites Share
- Description
Product Detail
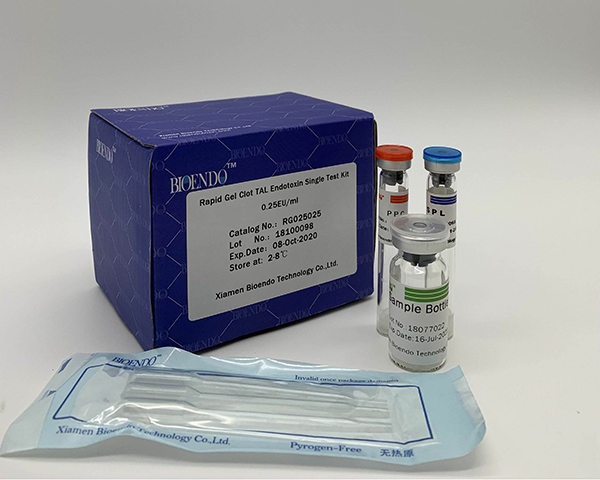
Limulus Amebocyte Lysate assay LAL reagent Single Test Vial gel clot method
Limulus Amebocyte Lysate assay LAL reagent Single Test Vial gel clot method
We are the first TAL reagent provider at China. The first TAL lysate provider licensed at China FDA. Gel Clot single test does not require pyrogen free test tubes. Add the test sample and read the positive or negative results in a simple step. The testing method conforms to Pharmacopoeia Bacterial Endotoxins Test. Our gel clot reagents are strong resistance to interferences, has the widest range of sensitivities.
1. Product information
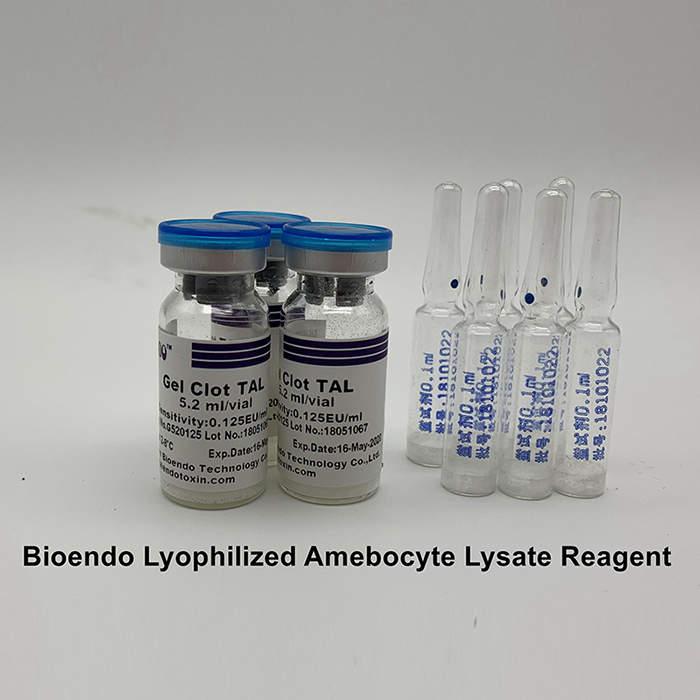
LAL gel clot test is the simple endotoxin testing by the forming of the gel clot by endotoxin and LAL lysate. Gel Clot LAL reagent single test vial is the single test Gel Clot LAL reagent sealed under vacuum condition in a standard LAL reaction tube. Users add 0.2ml sample to dissolve the LAL reagent and do the test in a single step. This method comforts to Pharmacopoeia Bacterial Endotoxins Test. The cost per test will reduce because there is no need to prepare depyrogenated endotoxin free test tubes. We provide the gel clot single test vial in sensitivity 0.015EU/ml to 0.25EU/ml. The gel clot LAL kit with Control Standard Endotoxin is also available.
2. Product parameter
One test per tube
Sensitivities: 0.015, 0.03, 0.06, 0.125, 0.25 EU/ml
Sample requirement: 0.2ml per test.
3.Product feature and application
Single step endotoxin detection without expensive endotoxin assay instruments. Suitable for end-product endotoxin testing before product released. Product sensitivity standardized with USP Standard Endotoxin.
Operation chart

4. Ordering information
Gel Clot LAL Single Test Vial, 50 test tubes/pack,
Catalog Number | Sensitivity (EU/ml) |
G020015 | 0.015 |
G020030 | 0.03 |
G020060 | 0.06 |
G020125 | 0.125 |
G020250 | 0.25 |
5. Product Qualification

6. Ordering FAQ
Product condition:
The LAL reagent sensitivity and the Control Standard Endotoxin potency are assayed against USP Reference Standard Endotoxin. The LAL reagent kits come with product instruction, Certificate of Analysis, MSDS.
How to order:
Please contact sales@houshiji.com to order the LAL reagents endotoxin detection kit. There is no minimal order volume required. The price depends on the order volume. We provide distributor discount for our distributors. The price is in US dollars. The final cost will be the order price plus the shipping cost. We need to know the shipping item to calculate the shipping weight.
Payment Method:
We required 100% prepayment for all orders. We accept T/T bank transfer or Paypal. If pay by Paypal 5% additional charge is required.
Shipping Condition:
Once we received the payment, we will ship in two days. Shipping is usually by air, it takes less than one week to reach the location. LAL Reagent is stable at controlled room temperature for more than two weeks. We usually ship the reagent at room temperature. After receipting LAL Reagent, long term storage at 2-8? is required.
Storage Condition:
The LAL reagent is stable for more than two years at 2-8?. Store LAL reagent at 2-8? , avoid light. LAL Reagent Water is stable for more than three years at 2-20? .
Note:
LAL Reagent ( TAL Reagent ) from our company are formulated from the aqueous extract of circulating amebocytes of Chinese horseshoe crab (Tachypleus tridentatus).
- PAUT Flat Scraper Bottom Discharging Automatic Centrifuges 1 Pieces / (Min. Order)
- PAUT Flat Scraper Bottom Discharging Automatic Centrifuges 1 Pieces / (Min. Order)
- PGZ Type Flat Scraper Discharging Automatic Centrifuges 1 Pieces / (Min. Order)
- Double Vertical Ring Pulsating High Gradient Magnetic Separator 1 Pieces / (Min. Order)
- Residual YCH-4 Fluid Recovery Apparatus 1 Pieces / (Min. Order)
- KTS670 Diagnostic Apparatus 1 Pieces / (Min. Order)
- GK Series Horizontal Scraper Discharging Separator 1 Pieces / (Min. Order)
- New arrival high brightness par30 e27 30w led spotlight 35w 45w par20/par30/par38 1 Pieces / (Min. Order)
- General Standard Three Axis Magnetic Fluxgate Sensor (Model: HS-MS-FG3S) 1 Pieces / (Min. Order)
- 7 Segment LCD Display OEM LCD display LED dot matrix 7 segment Character LED display 1 Pieces / (Min. Order)
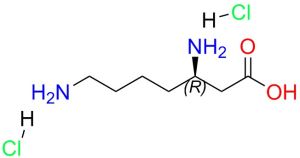
- D-beta-homolysine-2HCl , 1301706-69-3 , (R)-3,7-Diaminoheptanoic acid dihydrochloride 1 Pieces / (Min. Order)
- The Regenerated Catalyst Of Hydrogen Peroxide By Anthraquinone 1 Pieces / (Min. Order)
- Guest stars! UNDEAD man hunting Adonis cosplay costume 1 Pieces / (Min. Order)
- End seraph Nagoya Kessen hen 100 night Michaela cosplay costume 1 Pieces / (Min. Order)
- Molecular Distillation Apparatus 1 Pieces / (Min. Order)
- Bluetooth 3.0 Portable Wireless Speaker TF USB Aux Radio Built-in Mic Dual Speaker Bass Sound 1 Pieces / (Min. Order)
- DC12V 24V 4V110-06 3V110-06 5 Way 2 3 Way 2 Single Solenoid Pneumatic Air Valve 1/8" 1 Pieces / (Min. Order)
- New Generation High Frequency Color Ultrasound Diagnostic System for Vet with Varies Probes 1 Pieces / (Min. Order)
- Asphalt Fraass Breaking Point Apparatus 1 Pieces / (Min. Order)
- Efficient Centrifugal Rotary Small Scraper Thin Film Evaporator/Falling Film Evaporator 1 Pieces / (Min. Order)
 Menu
Menu
















 Favorites
Favorites

 Frequent updates ensuring high quality data
Frequent updates ensuring high quality data
 Over 5000 customers trust us to help grow their business!
Over 5000 customers trust us to help grow their business!


 Menu
Menu