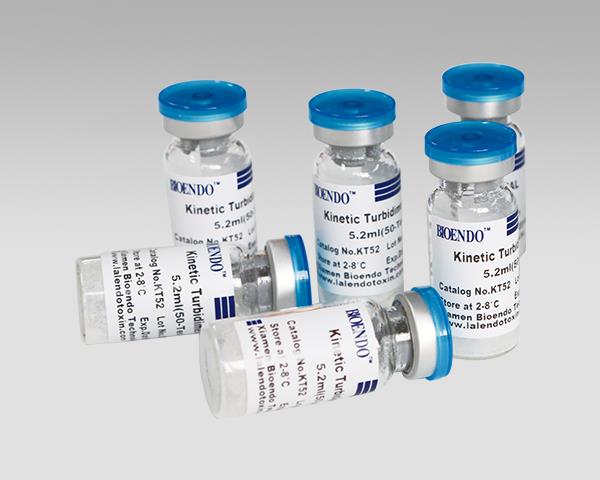
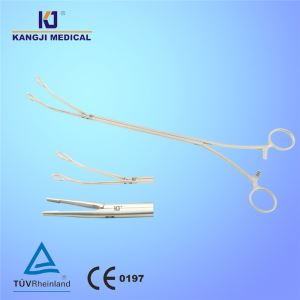
LAL Lysate Vials for Endotoxin Testing Kinetic Turbidimetric Method
-
Min Order
1
-
Product Unit
Pieces
-
Origin
China Mainland
-
Payment


- Contact Now Start Order
- Favorites Share
- Description
Product Detail
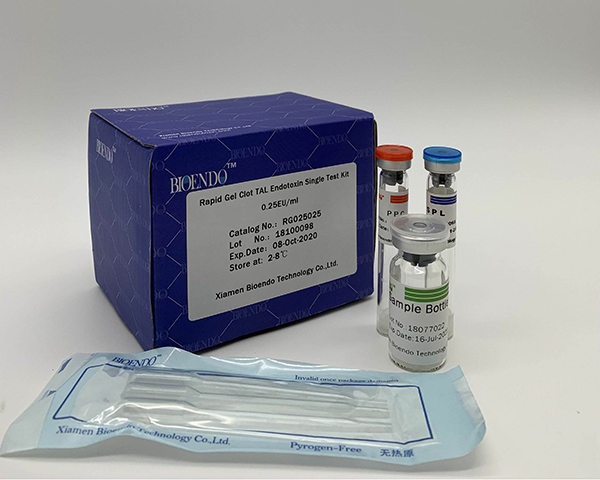
LAL lysate vials for endotoxin testing kinetic turbidimetric method
LAL lysate vials for endotoxin testing kinetic turbidimetric method
We supply kinetic turbidimetric LAL lysate for pharmaceutical and medical device industries for testing the endotoxin level of the injectable drugs and implantable medical devices at China. In the past 35 years, we devoted ourselves to the research of LAL endotoxin testing technology, to make sure the quality of drug products and medical devices, allows the monitoring of the endotoxin level during production process.
1. Product introduction
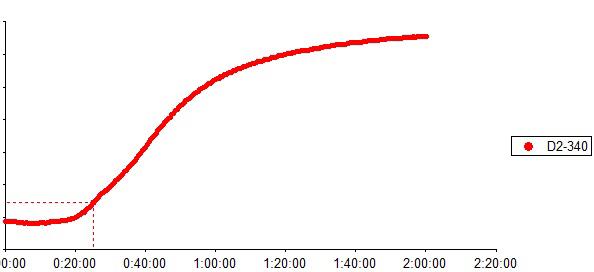
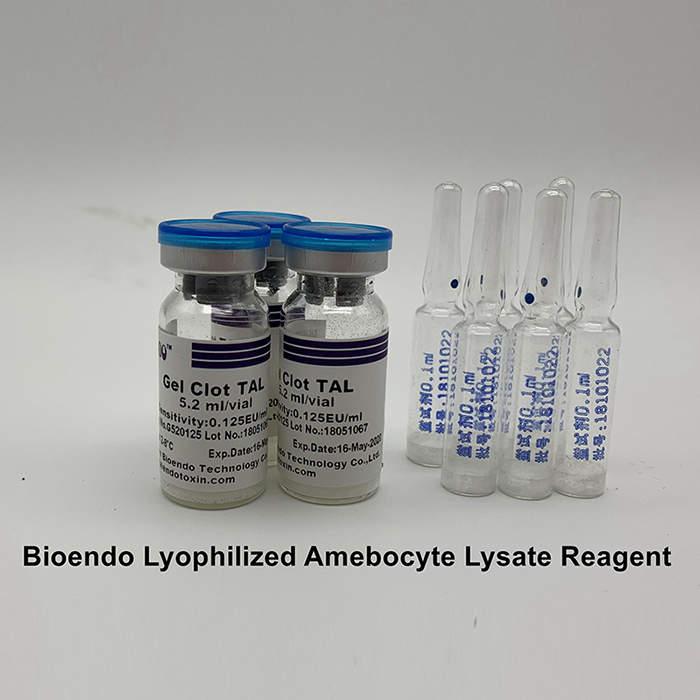
LAL reagent is the extract from horseshoe crab blue blood lysate. The kinetic LAL assay a simple measurement for endotoxin quantification. Based on the principle that in the presence of endotoxin LAL becomes turbid, the increase rate of turbidity is proportional to the endotoxin concentration of the sample, the endotoxin is quantified. The detection limit for the assay kit is 0.005EU/ml and the detection range is up to four orders of magnitude. Using kinetic microplate reader Elx808IULALXH and TALgent software, kinetic turbidimetric assay automatic quantify endotoxins in a single step for 96 tests at a time. The test efficiency could be improved dramatically with the help of multi-channel pipettor or automatic sample processing device. Kinetic turbidimetric assay is applicable to the pharmaceutical industry endotoxin monitoring in the manufacturing process, including raw materials, water for injection, intermediate products and end products endotoxin tests. We provide kinetic turbidimetric LAL reagent in 0.5ml, 1.7ml, 2.2ml and 5.2ml per vials.
The reagent is recommended to use for Water for Injection endotoxin testing. LAL endotoxin assay kits include control standard endotoxin, LAL reagent water, pyrogen-free pipettor tips, glass test tube for endotoxin dilution is also available.
2. Product parameter:
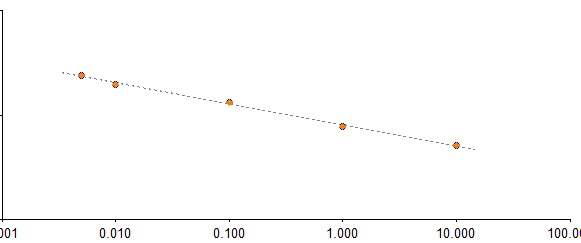
Assay range: 0.005-50EU/ml
3. Product application
End-product endotoxin (pyrogen) qualification, Water for injection endotoxin assay, raw material endotoxin testing or endotoxin level monitoring during manufacturing process for pharmaceutical companies or medical devices manufacturers.
Operation chart
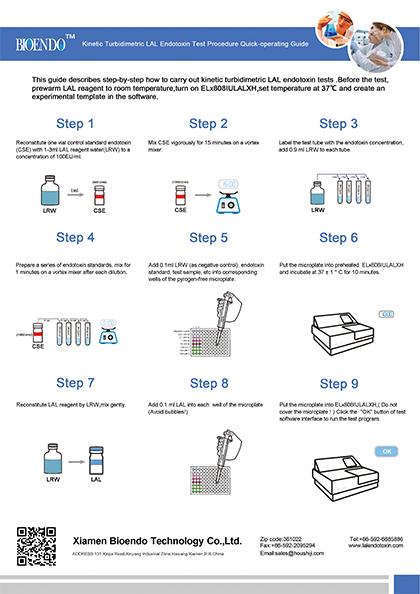
Standard curve:
4. Order information:
ml/vial | Tests/vial | Vials/pack | |
KT05 | 0.5 | 4 | 10 |
KT17 | 1.7 | 15 | |
KT22 | 2.2 | 20 | |
KT28 | 2.8 | 26 | |
KT52 | 5.2 | 50 |
5. Product Qualification

6. Ordering FAQ
Product condition:
The LAL reagent sensitivity and the Control Standard Endotoxin potency are assayed against USP Reference Standard Endotoxin. The LAL reagent kits come with product instruction, Certificate of Analysis, MSDS.
How to order:
Please contact sales@houshiji.com to order the LAL reagents endotoxin detection kit. There is no minimal order volume required. The price depends on the order volume. We provide distributor discount for our distributors. The price is in US dollars. The final cost will be the order price plus the shipping cost. We need to know the shipping item to calculate the shipping weight.
Payment Method:
We required 100% prepayment for all orders. We accept T/T bank transfer or Paypal. If pay by Paypal 5% additional charge is required.
Shipping Condition:
Once we received the payment, we will ship in two days. Shipping is usually by air, it takes less than one week to reach the location. LAL Reagent is stable at controlled room temperature for more than two weeks. We usually ship the reagent at room temperature. After receipting LAL Reagent, long term storage at 2-8? is required.
Storage Condition:
The LAL reagent is stable for more than two years at 2-8?. Store LAL reagent at 2-8? , avoid light. LAL Reagent Water is stable for more than three years at 2-20? .
Note:
LAL Reagent ( TAL Reagent ) from our company are formulated from the aqueous extract of circulating amebocytes of Chinese horseshoe crab (Tachypleus tridentatus).
- Copper Clad Steel Wire Annealing Furnace 1 Pieces / (Min. Order)
- Transparent Pressure-sensitive Adhesive 1 Pieces / (Min. Order)
- Sparks Direct-reading Spectrometer 1 Pieces / (Min. Order)
- Expanded PTFE Joint Sealant Tapes 1 Pieces / (Min. Order)
- Expanded PTFE Joint Sealant 1 Pieces / (Min. Order)
- Ethiopia Wheat Flour Process 20T Per 24h 1 Pieces / (Min. Order)
- Alarm Monitoring Service 1 Pieces / (Min. Order)
- Debakey Grasping Forceps 1 Pieces / (Min. Order)
- High Capacity Profile Single Screw Extruder 1 Pieces / (Min. Order)
- Women Custom Cheap Short Sleeve T shirt 1 Pieces / (Min. Order)
- 18.TECHHOODIE.MEN 1 Pieces / (Min. Order)
- Men's Sweatpants 1 Pieces / (Min. Order)
- Men Fur Zip Hd 1 Pieces / (Min. Order)
- Jrs Zip Hd 1 Pieces / (Min. Order)
- Men T Shirt 1 Pieces / (Min. Order)
- JRS FUR HOOD 1 Pieces / (Min. Order)
- Boys Full Zip Hd 1 Pieces / (Min. Order)
- Assembly Parts Checking Fixture for Wheel Casing 1 Pieces / (Min. Order)
- Assembly Parts Checking Fixture for Car Light 1 Pieces / (Min. Order)
- Company Price Single Girder Bridge Crane 1 Pieces / (Min. Order)
 Menu
Menu


















 Favorites
Favorites

 Frequent updates ensuring high quality data
Frequent updates ensuring high quality data
 Over 5000 customers trust us to help grow their business!
Over 5000 customers trust us to help grow their business!


 Menu
Menu