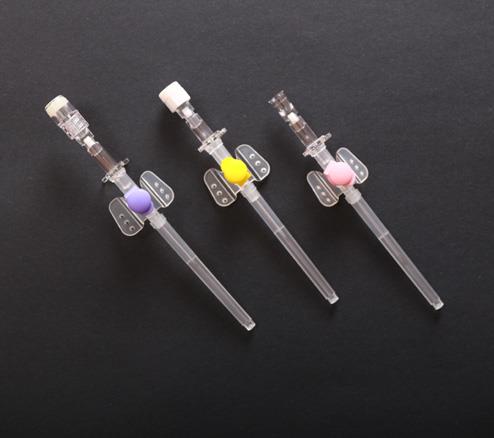
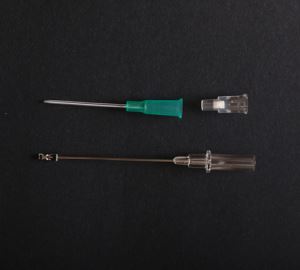
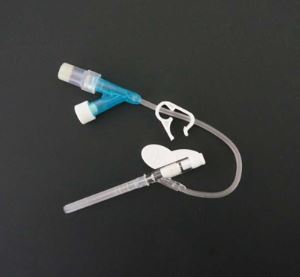
IV Cannula Or Intravenous Catheter With Injection Port
-
Min Order
1
-
Product Unit
Pieces
-
Origin
China Mainland
-
Payment


- Contact Now Start Order
- Favorites Share
- Description
Product Detail
IV cannula or intravenous catheter with injection port
IV cannula with injection port is one popular type IV cannula in peripheral venous infusion.IV cannula with injection port can also be used as veterinary IV catheter.Our intravenous catheter is CE certied.
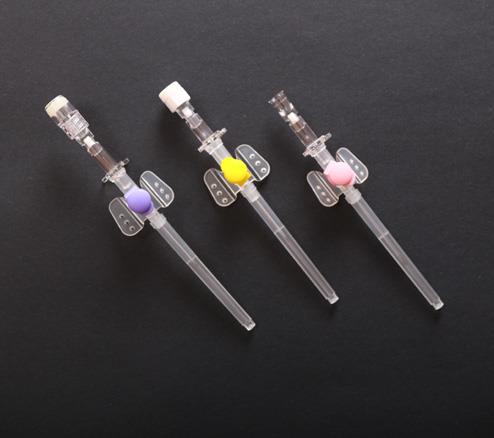 | IV cannula with injection port is composed of protective tube, catheter, unidirectional valve, fixed rivet for tube, injection port cap, winged catheter seat, needle tube, needle tube seat, exhaust joint and spiral cap. |
The following is the description and requirement of IV cannula raw material of different components:
1.Needle peripheral catheters (the catheter) of intravenous catheter is made from Fluorinated Ethylene Propylene (FEP). Catheter of IV butterfly catheter or veterinary IV catheter should be no biological hazards.
2.The needle tube is made of 06Cr19Ni10 (SUS304) material specified in GB/T 1220.
3.Needle seat is made of polycarbonate (PC) material.
4.Raw material of unidirectional valve is medical silicone rubber.
5.Raw material of winged catheter seat, injection port cap, exhaust joint and spiral cap is medical polypropylene (PP).
6.Raw material of protective tube is medical polyethylene (PE) material.
Quality standards:
1. Needle tube should have good rigidity and toughness. The tip of the needle should be sharp and without edges, burrs and crooked hooks.
2. Needle should not loose in the needle seat.
3. Protective tube should not fall off naturally after being assembled with the catheter seat.
4. The metal parts of IV catheters should have no trace of corrosion.
5. After being sterilized by EO gas, the residue should be less than 10µg/g.
Applications:
IV cannula is suitable for peripheral venous infusion, and the retention time is not more than 72 hours.
The usage shall be stopped immediately if there is any local reaction to irritation.
Warnings
1. If there is any liquid leakage , the usage shall be stopped.
2. IV cannula with vreases on the catheter shall not be used.
3. Intravenous cannula with signs of fracture shall not be used.
Notes:
1.The products can only be used by trained doctors or nurses.
2.The small packing should be checked before use. If breakage is found, the IV cannula shall not be used.
3.Puncture points should be checked regularly.If there are signs of local or body infection, Iv cannula should be stopped immediately.
4.After being used, the needle and catheter shall be destroyed and put into special waste box.
5.This product is disposable product and repeated usage is forbidden.
6.In terms of storage according to regulated conditions, the product validity time is three years.
Storage method
Iv catheters shall be stored in a cool, dry, clean, well ventilated, and non corrosive environment.
Specifications: 14G,16G,18G,20G,22G,24G,26G
Package:
1 pc in a hard blister pack
50 pcs per paper box
20 boxes per carto
Size: 14G,16G,18G,20G,22G,24G,26G
-
Electromagnetic Heating Planet Stir-frying Pans
US $9.84-13.6 / Price
1 Pieces / (Min. Order) -
Electronic Monitoring Code Inkjet Printer
US $9.85-19.56 / Price
1 Pieces / (Min. Order) - Electronic Weighing And Packing Line 1 Pieces / (Min. Order)
- Intelligent Sampling Weighing Instrument 1 Pieces / (Min. Order)
- Imitation Green Bronze Inscriptions 1 Pieces / (Min. Order)
- HH Electric Heating Constant Temperature Oil Bath 1 Pieces / (Min. Order)
- Electric Heating Constant Temperature Incubator 1 Pieces / (Min. Order)
- Flanged Disc Bearing Unit FD208R1 1 Pieces / (Min. Order)
- Factory low price nylon eyelash lace fabric for women's garment 1 Pieces / (Min. Order)
- Single or Double Heads Hydraulic Drawing Bench for Tube and Sheet,Full Automatic 1 Pieces / (Min. Order)
- Egyptians White Kidney Beans in Brine Canned Beans 1 Pieces / (Min. Order)
- Adjustable Multi-vent Venturi Mask Kit 1 Pieces / (Min. Order)
- Disposable CPAP Mask And Headgear And Tubing 1 Pieces / (Min. Order)
- 14G,16G,18G,20G,22G,24G,26G IV Catheter Pen Type 1 Pieces / (Min. Order)
- IV Butteryfly Winged Peripheral Catheter 1 Pieces / (Min. Order)
- Safety Lock IV Catheter Or Cannula 1 Pieces / (Min. Order)
- Y-type New IV Catheter Disposal With Extension 1 Pieces / (Min. Order)
- Portable Laboratory Colorimeter 1 Pieces / (Min. Order)
- Portable Nitrite Nitrogen Colorimeter 1 Pieces / (Min. Order)
 Menu
Menu















 Favorites
Favorites

 Frequent updates ensuring high quality data
Frequent updates ensuring high quality data
 Over 5000 customers trust us to help grow their business!
Over 5000 customers trust us to help grow their business!


 Menu
Menu