

Singclean China Adhesion Barrier Gel Suppliers
-
Min Order
1
-
Product Unit
Pieces
-
Origin
China Mainland
-
Payment


- Contact Now Start Order
- Favorites Share
- Description
Product Detail
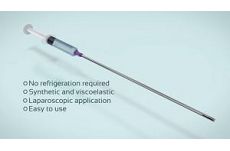
1. What is Singclean® Adhension Barrier Gel?
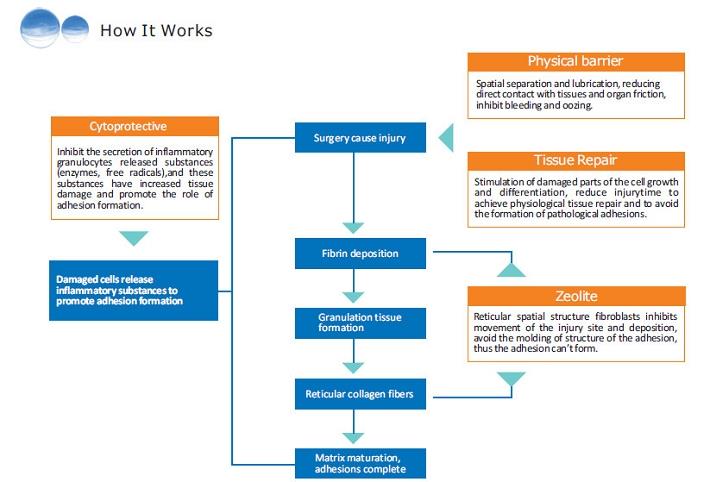
This product is a biocompatible, absorbable, flowable gel composed of hyaluronic acid sodium salt and physiologically balanced salt. With no sulfate groups in its molecule and no covalent binding with the protein, it can exist as free chain in the body for lubrication, moisture preservation and mechanical isolation, preventing adhesions.
Specification
Singclean® Adhesion barrier Gel | |
Volume | Concentration |
1.0ml | 10mg/ml |
2.0ml | 10mg/ml |
2.5ml | 10mg/ml |
3ml | 10mg/ml |
4ml | 10mg/ml |
5ml | 10mg/ml |
10ml | 10mg/ml |
20ml | 10mg/ml |
2. When should consider Singclean® Adhesion barrier Gel?
Used for the prevention and reduction of the incidence, extent, and severity postoperative adhesions of the abdominal and pelvic surgery.
3. How to use Singclean® Adhesion barrier Gel:

4. Important Safety Information
a. The product is sterilized and free from pyrogenicity, and must be operated in a sterile environment;
b. The product is single use only. Do not use if sterile packaging has been damaged
c. This product must not be in contact with drugs containing geramine chloride in order to avoid chaos
d. Must not be used for intravenous injection
5. Why should use Singclean® Adhesion barrier Gel?
The Singclean® Adhesion barrier gel has a longer retention time (7-14days) at the site of injury, which does not cause inflammation, not inhibit wound healing
The Singclean® Adhesion barrier gel is the most effective in preventing postoperative adhesions in the abdominal cavity and pelvic cavity. It can mechanically isolate the injured site and prevent the formation of fibrin reticular formation between the surface of the tissue.
The Singclean® Adhesion barrier gel is an effective way to prevent or reduce adhesions.
Singclean® Adhesion barrier Gel is proven to reduce the incidence, extent, and severity of adhesions in patients undergoing open or abdominal and pelvic surgery
6. Warnings and Precautions
- Confirm that the surgical site is free of excessive bleeding. Excessive bleeding should be controlled prior to Singclean® Adhesion barrier Gel instillation.
- Singclean® Adhesion barrier Gel does not have intrinsic bacteriostatic or bactericidal activity. It is not bacteriostatic toward pre-existing infections, nor does it prevent the occurrence of new infections. In the case of pre-existing infections, appropriate treatment should be instituted.
- The concomitant use of Singclean® Adhesion barrier Gel with other anti-adhesion device has not been evaluated.
- Singclean® Adhesion barrier Gel has not been evaluated with a dose of over 2 ml/kg of body weight. It is not recommended to use Singclean® Adhesion barrier Gel over a dose of 2 ml/kg of body weight unless at the discretion of the physician.
- Singclean® Adhesion barrier Gel has not been evaluated in patients affected by malignant tumors. Preclinical evidence has shown that the products have no influence on neoplastic diffusion.
- Data on the use of Singclean® Adhesion barrier Gel on pregnant women are not available. The use of the products is not recommended in this condition. It is also recommended to avoid pregnancy during the first complete menstrual cycle subsequent to the treatment.
- Do not inject intravascularly.
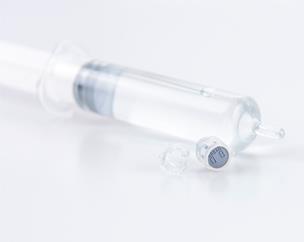
- Singclean® Adhesion barrier Gel is provided in single-use, pre-filled syringes. The syringe content only is provided sterile. The external surface of the syringe has a very low controlled bio-burden. The syringe is packaged into a protective blister tray or Tyvek pouch to prevent the contamination of its outer surface and to allow the use of the devices in the surgical theatre. Do not re-sterilize the syringe content.
- It is recommended to use the product immediately after opening the pouch.
- All the assembling operations of the device must be performed in the surgical theatre.
- The syringe is single-use; any non-used product must be discarded accordingly. The reuse of the product may cause serious infection.
- The empty containers have to be discarded accordingly.
- Keep out of reach of children.
- If the syringe and /or protective pouch or the blister is damaged, do not use the product and contact local distributor.
- Do not use the product after the expiration date.
- Singclean® Adhesion barrier Gel must be used according to the instruction for use. Read instructions prior to use.
7. Company Profile
Hangzhou Singclean Medical Products Co., Ltd., established in March 2003 with registered capital of 80,000,000 RMB, is a national high and new technology enterprise integrating the R&D, production and sales of medical biomaterials. With well-qualified staff, thorough quality management system and first-class facilities, the company ensures the continuous production of medical devices and drug injection products of Class III that confirm to the laws and regulations of the State Food and Drug Administration (SFDA) and EU MDD.


8. Certification
ISO13485, ISO9001, SGS



9. Packaging & Shipping

10. Our Services
a. Supply different product made from Sodium Hyaluronate with high quality
b. 24 hours online services
c. OEM,ODM and Customized is available
d. Quickly delivery time
e. Quickly and professional after sales service
11. FAQ
Q: What is hyaluronic acid?
A: Hyaluronic acid (HA) is a unique linear macromolecular mucopolysaccharide. It consists of glucuronic acid and n-acetyl glucosamine disaccharide units alternately connected together, the molecular formula is (C14H21NO11)n. The molecular weight changes between 105~107 based on different tissue of origin. HA is widely distributed in animal and human connective tissue extracellular matrix, with higher concentration in vitreous, umbilical cord, skin, cartilage and synovial fluid. We use bacterial fermentation to product HA material to avoid the risk of carrying virus and media from animal tissues or organs. HA is different from other natural mucopolysaccharide; HA molecule does not contain sulfate groups. It is also not covalently bound protein, and can exist free in the body as freedom chain. It is high viscosity gel for the anti-adhesion treatment.
Q: Normally, how many methods for sterilizing? What is the application range for each method? Which method we use for our products?
A:The common sterilizing methods are moist heat sterilization, irradiation sterilization, eo sterilization (Ethylene oxide) and aseptic processing (Process control). Moist heat sterilization is used widely, Singclean anti-adhension Gel also use this method.
Q: Which certificates you have?
A:We already get CE, ISO13485,CFDA, SGS and ISO9001.
- DOCSIS 3.0 RJ45 Port Coaxial Cable Modem DB-CM301 1 Pieces / (Min. Order)
- Reverse Polarity BNC Female To BNC Male Adapter (CT5075B) 1 Pieces / (Min. Order)
- Original 110V AC Power Adapter Wall Travel Charger Cord For NDSI/NDSI XL And 3DS - US Plug 1 Pieces / (Min. Order)
- OHP2003(Student’s Cute Carton Pencil Case Paper Box) 1 Pieces / (Min. Order)
- 0HP2004(Student’s Cat Or Puppy Or Bunny Or Bear Carton Pencil Case Paper Box 1 Pieces / (Min. Order)
- 2015 Buiness Ideals 50 BOPP Woven Packaging Bag For Organic Rice Products 1 Pieces / (Min. Order)
- High Quality 10 Drawers Rolling Tool Chest Cabinet With Plastic Worktop 1 Pieces / (Min. Order)
- High Quanlity 7-Drawer Heavy Duty Ball-Bearing Workstation With Extra Stroage Tool Chest 1 Pieces / (Min. Order)
- Full Covered Titanium Alloy Tempered Glass Screen Protector 1 Pieces / (Min. Order)
- Standard BHC-1300IIA2 Biological Safety Cabinets 1 Pieces / (Min. Order)
- Standard BHC-1300IIB2 Biological Safety Cabinets 1 Pieces / (Min. Order)
- 5 Ton Heavy Duty Manual Pallet Truck 1 Pieces / (Min. Order)
- Titanium Deep-sea Pressure Tank products 1 Pieces / (Min. Order)
- CNC spring forming machine for 3D precision complex wire products 1 Pieces / (Min. Order)
- Puffa Puffy Jacket Windbreakers Customized Bespoke Personalized Jacket Windproof Jacket For Kids 1 Pieces / (Min. Order)
- Goat Milk Body Scrub 1 Pieces / (Min. Order)
- Goat Milk Soap 1 Pieces / (Min. Order)
- Goat Milk Bath Salts 1 Pieces / (Min. Order)
- Goat Milk Conditioner 1 Pieces / (Min. Order)
- Goat Milk Hand Wash 1 Pieces / (Min. Order)
 Menu
Menu























 Favorites
Favorites
 Frequent updates ensuring high quality data
Frequent updates ensuring high quality data
 Over 5000 customers trust us to help grow their business!
Over 5000 customers trust us to help grow their business!


 Menu
Menu