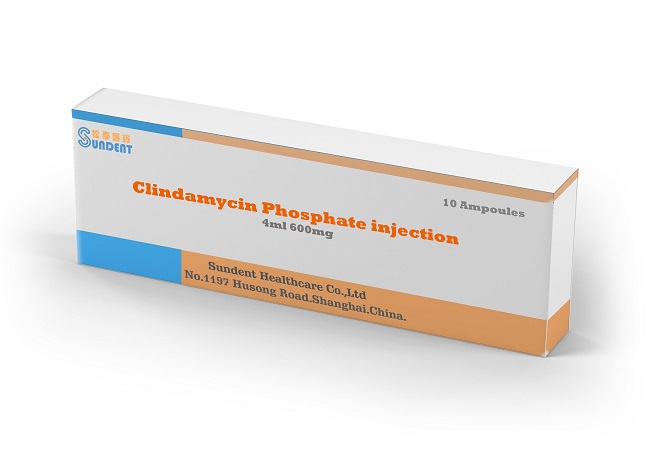
Clindamycin Injection
-
Min Order
1
-
Product Unit
Pieces
-
Origin
China Mainland
-
Payment


- Contact Now Start Order
- Favorites Share
- Description
Product Detail
Clindamycin injection
Indication
Product Name: Clindamycin phosphate injection
Nombre del Producto:Fosfato de Clindamicina solución inyectable
Specification: 600mg/4ml
Package: 10amps/box, 50amps/box
Standard: USP & BP & CP
Posology and method of administration
Parenteral (IM or IV administration). Clindamycin 600mg/4ml Solution for Injection and Infusion must be diluted prior to I.V. administration and should be infused over at least 10-60 minutes.
Adults: Serious infections: 600mg-1.2g/day in two, three or four equal doses.
More severe infections: 1.2-2.7g/day in two, three or four equal doses.
Single I.M. injections of greater than 600mg are not recommended nor is administration of more than 1.2g in a single one-hour infusion.
For more serious infections, these doses may have to be increased. In life-threatening situations, doses as high as 4.8g daily have been given intravenously to adults.
Alternatively, the drug may be administered in the form of a single rapid infusion of the first dose followed by continuous IV infusion.
Children (over 1 month of age): Serious infections: 15-25mg/kg/day in three or four equal doses.
More severe infections: 25-40mg/kg/day in three or four equal doses. In severe infections it is recommended that children be given no less than 300mg/day regardless of body weight.
Elderly patients: The half-life, volume of distribution and clearance, and extent of absorption after administration of clindamycin phosphate are not altered by increased age. Analysis of data from clinical studies has not revealed any age-related increase in toxicity. Dosage requirements in elderly patients should not be influenced, therefore, by age alone.
Dosage in renal/hepatic impairment: clindamycin dosage modification is not necessary in patients with renal or hepatic insufficiency
Treatment for infections caused by beta-haemolytic streptococci should be continued for at least 10 days to guard against subsequent rheumatic fever or glomerulonephritis.
Contraindications
Clindamycin 600mg/4ml Solution for Injection and Infusion is contra-indicated in patients previously found to be sensitive to clindamycin, lincomycin or to any of the excipients.
Special warnings and precautions for use
Warnings
Clindamycin 600mg/4ml Solution for Injection and Infusion should only be used in the treatment of serious infections. In considering the use of the product, the practitioner should bear in mind the type of infection and the potential hazard of the diarrhoea which may develop, since cases of colitis have been reported during, or even two or three weeks following, the administration of clindamycin.
Studies indicate a toxin(s) produced by clostridia (especially Clostridium difficile) is the principal direct cause of antibiotic-associated colitis. These studies also indicate that this toxigenic clostridium is usually sensitive in vitro to vancomycin. When 125mg to 500mg of vancomycin are administered orally four times a day for 7-10 days, there is a rapid observed disappearance of the toxin from faecal samples and a coincident clinical recovery from the diarrhoea (where the patient is receiving cholestyramine in addition to vancomycin, consideration should be given to separating the times of administration).
Precautions
Caution should be used when prescribing Clindamycin 600mg/4ml Solution for Injection and Infusion to individuals with a history of gastro-intestinal disease, especially colitis.
Periodic liver and kidney function and haematology tests should be carried out during prolonged therapy. Such monitoring is also recommended in neonates and infants. Safety and appropriate dosage in infants less than one month old have not been established.
Prolonged administration of Clindamycin 600mg/4ml Solution for Injection and Infusion, as with any anti-infective, may result in super-infection due to organisms resistant to clindamycin. The use of Clindamycin 600mg/4ml Solution for Injection and Infusion may result in the overgrowth of non-susceptible organisms particularly yeasts.
Care should be observed in the use of Clindamycin 600mg/4ml Solution for Injection and Infusion in atopic individuals, particularly those with asthma.
Since Clindamycin 600mg/4ml Solution for Injection and Infusion does not diffuse adequately into cerebrospinal fluid, the drug should not be used in the treatment of meningitis.
Antibiotics can reduce the efficacy of the combined oral contraceptive pill. Additional contraceptive precautions should be taken during treatment and for up to seven days after stopping treatment.
This medicinal product contains 0.286mMol (or 6.57mg) sodium per ml of solution (prior to dilution). To be taken into consideration by patients on a controlled sodium diet.
Interaction with other medicinal products and other forms of interaction
Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. It should be used with caution, therefore, in patients receiving such agents.
Antagonism has been demonstrated between clindamycin and erythromycin in vitro. Because of possible clinical significance, the two drugs should not be administered concurrently.
Vitamin K antagonists
Increased coagulation tests (PT/INR) and/or bleeding have been reported in patients treated with clindamycin in combination with a vitamin K antagonist (e.g. warfarin, acenocoumarol and fluindione). Coagulation tests, therefore, should be frequently monitored in patients treated with vitamin K antagonists.
Related Keywords: We are considered as one of the leading manufacturers and suppliers of clindamycin injection. With the aid of our technologically advanced manufacturing section, we are able to offer customers clindamycin injection products which are tested on various quality parameters in order to supply flawless range at the customers' end.
-
Animal Ear Tag
US $5.27-14.93 / Price
1 Pieces / (Min. Order) - Natural And Pure Sealwort Extract 1 Pieces / (Min. Order)
- Epimedium Extract 1 Pieces / (Min. Order)
- Astragalus Extract 1 Pieces / (Min. Order)
- Cinnamon Bark Extract 1 Pieces / (Min. Order)
- Cnidium Seed Extract 1 Pieces / (Min. Order)
- Fenugreek Seed Extract 1 Pieces / (Min. Order)
- Lemon Balm Extract 1 Pieces / (Min. Order)
- Magnolia Bark Extract 1 Pieces / (Min. Order)
- Radix Isatidis Extract 1 Pieces / (Min. Order)
- Astragalus Extract 1 Pieces / (Min. Order)
- Epimedium Extract 1 Pieces / (Min. Order)
- Goji Berry Extract 1 Pieces / (Min. Order)
- Marigold Extract 1 Pieces / (Min. Order)
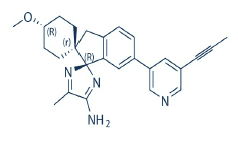
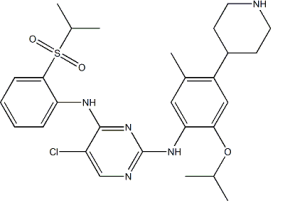
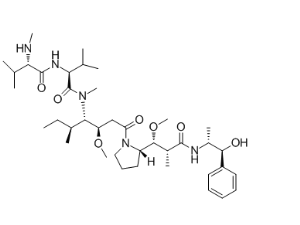
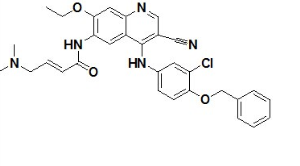
- CC-223 CAS:1228013-30-6 1 Pieces / (Min. Order)
- CEP 32496 CAS:1188910-76-0 1 Pieces / (Min. Order)
- AZD3293 CAS:1383982-64-6 1 Pieces / (Min. Order)
- Ceritinib CAS:1032900-25-6 1 Pieces / (Min. Order)
- Monomethylauristatin E CAS:474645-27-7 1 Pieces / (Min. Order)
- Neratinib CAS:698387-09-6 1 Pieces / (Min. Order)
 Menu
Menu














 Favorites
Favorites



 Frequent updates ensuring high quality data
Frequent updates ensuring high quality data
 Over 5000 customers trust us to help grow their business!
Over 5000 customers trust us to help grow their business!


 Menu
Menu